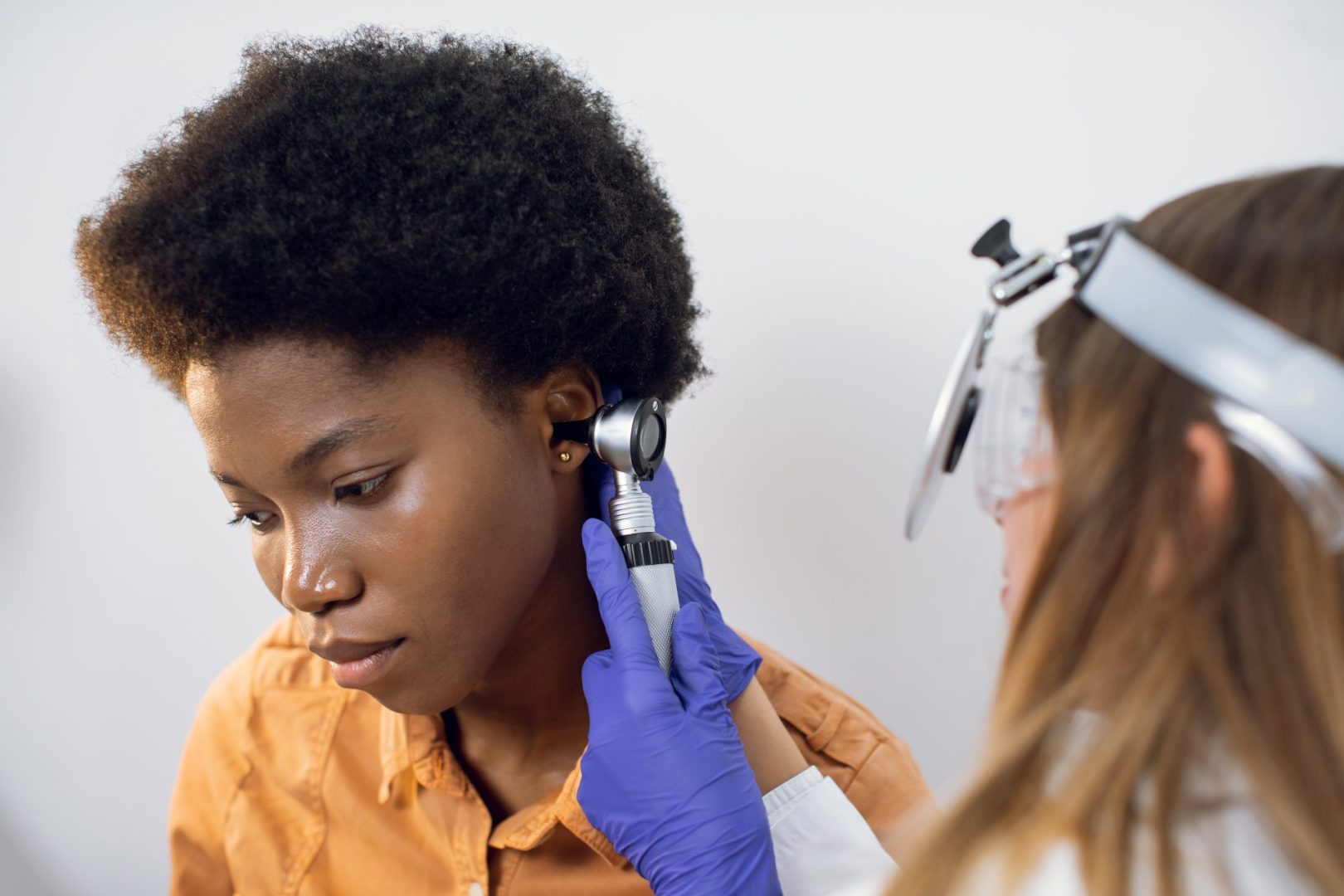
Progressive genetic hearing loss is a condition where hearing fades gradually over time due to inherited factors. Unlike sudden hearing loss from injury or infection, this type begins subtly—often unnoticed—and worsens as years pass. It’s tied to changes in specific genes that disrupt the delicate machinery of the inner ear or the auditory pathways to the brain. This article unpacks five essential truths about the condition, shedding light on its origins, progression, and the promise of emerging solutions, all while keeping the focus on what it means for those affected.
It stems from inherited gene changes
At its core, progressive genetic hearing loss arises from alterations in DNA passed down through families. These changes affect genes crucial to hearing, like those responsible for the tiny hair cells in the cochlea that turn sound waves into electrical signals for the brain. Some mutations weaken these cells over time, while others impair the nerves that carry sound information. The condition can follow different inheritance patterns—autosomal dominant, where one altered gene from a parent is enough, or autosomal recessive, requiring two. Mitochondrial inheritance, linked to DNA in cell energy hubs, also plays a role, often affecting both hearing and other systems. This genetic root sets it apart from age-related or noise-induced hearing loss, though the lines can blur when environmental factors amplify the decline.
Symptoms creep in over years
The hallmark of this condition is its slow onset. Early signs might be faint—trouble catching high-pitched sounds like birds chirping or children’s voices. Over time, the loss spreads to lower frequencies, making conversations harder to follow, especially in noisy places like cafes or crowded rooms. For some, it starts in childhood, progressing from mild to severe by adulthood. In others, it waits until later decades, mimicking age-related decline but driven by a genetic script. Balance issues or tinnitus—a persistent ringing—can tag along if the inner ear’s sensory structures falter. Because it unfolds gradually, many adapt unconsciously, turning up the TV or leaning closer, delaying recognition until the impact is undeniable.
Over 150 genes are linked to it
Research has pinpointed more than 150 genes tied to hearing loss, many of which can lead to a progressive form. Some, like GJB2, affect proteins that glue inner ear cells together, weakening over time. Others, such as MYO7A, disrupt the hair cells’ ability to flex and signal, causing a slow breakdown. Mitochondrial genes, like those in the 12S rRNA region, accumulate damage with age, hastening the decline. Each gene tells a unique story—some cause hearing loss alone, while others pair it with vision problems or heart issues, as in syndromes like Usher or Jervell and Lange-Nielsen. This vast genetic web explains why the condition varies so widely in when it starts, how fast it moves, and how severe it gets.
It’s more common than you think
Hearing loss touches millions globally, and genetics drives a hefty share. Up to 60% of hearing loss present at birth or in early childhood traces back to DNA, with many cases turning progressive as kids grow. For adults, over 25% of worsening hearing has a hereditary thread, often tangled with lifestyle factors like noise exposure. In families with a history, the odds climb—siblings or children of someone affected face a higher chance, depending on the inheritance pattern. Even without a clear family trail, spontaneous gene changes can spark it, making it a hidden player in more lives than statistics might suggest. As populations age and awareness grows, its reach becomes harder to ignore.
Hope lies in gene therapy advances
The future for progressive genetic hearing loss isn’t all dim. Science is pushing boundaries with gene therapy—techniques to fix or replace faulty genes. Early trials in animals, like mice with human-like hearing defects, have restored sound detection by delivering healthy gene copies to the inner ear. Tools like CRISPR, which edits DNA with precision, show promise in halting progression by correcting mutations before damage spreads. For some conditions, like otoferlin-related loss, therapies are inching toward human testing, aiming to revive hair cell function. Challenges remain—getting treatments to the right cells safely and timing them before hearing fades too far—but each step forward hints at a day when this slow fade might be paused or reversed.
Progressive genetic hearing loss weaves a complex tale of biology and time. It starts with a genetic glitch, unfolds through years of subtle shifts, and draws from a sprawling cast of genes. Its prevalence underscores a quiet epidemic, often masked by louder culprits like aging or noise. Yet, the horizon glimmers with possibility as researchers unravel its code and test ways to rewrite it. For now, tools like hearing aids and cochlear implants bridge the gap, amplifying what’s left. But understanding its roots—those five critical facts—offers more than facts. It’s a map to navigate the silence, and maybe, one day, to turn it back into sound.