Pancreatic cancer remains one of the most challenging cancers to detect early, contributing to its reputation as a silent disease with poor survival rates. The pancreas—a leaf-shaped organ tucked behind the stomach—performs crucial digestive and hormonal functions while remaining hidden from easy examination. Understanding the warning signs, particularly those that appear earliest, creates opportunities for timely intervention when treatment options offer the greatest benefit.
Why pancreatic cancer often escapes early detection
The pancreas’s location deep within the abdomen creates significant challenges for early detection. Nestled behind the stomach and surrounded by other organs, the pancreas cannot be felt during routine physical examinations and remains invisible to typical screening methods used for other cancers.
This vital organ serves dual functions that affect both digestion and blood sugar regulation. The exocrine functions produce digestive enzymes that break down proteins, fats, and carbohydrates, while endocrine functions regulate blood sugar through insulin and glucagon production. Different tumor types affect these distinct functions, creating varied symptom patterns.
Most pancreatic cancers (about 95%) develop in the exocrine tissue, with adenocarcinoma being the most common type. These tumors often begin in the pancreatic ducts and can grow significantly before causing noticeable symptoms. The remaining 5% develop in endocrine-producing cells, forming pancreatic neuroendocrine tumors that sometimes produce excess hormones, potentially creating earlier warning signs.
The pancreas has substantial functional reserve capacity, meaning it can continue performing adequately even when significant portions become damaged or diseased. This redundancy, while beneficial for maintaining function during minor injuries, allows tumors to grow considerably before affecting organ function enough to cause noticeable symptoms.
Current routine screening tests for pancreatic cancer don’t exist for the general population, unlike mammograms for breast cancer or colonoscopies for colorectal cancer. Imaging studies like CT scans or MRIs can detect pancreatic tumors but aren’t practical for routine screening due to cost, radiation exposure, and limited sensitivity for very small lesions.
Unexplained weight loss: A primary warning sign
Among the various warning signs, unexplained weight loss often represents one of the earliest and most common symptoms of pancreatic cancer, occurring in up to 85% of cases by the time of diagnosis.
This weight loss typically occurs without intentional dietary changes or increased physical activity—key characteristics that distinguish it from healthy weight loss. Many patients report losing 5-10 pounds or more within a few months without trying, often despite maintaining or even increasing their food intake.
Several mechanisms contribute to this weight loss. The cancer cells themselves require significant energy for their rapid multiplication, essentially consuming calories that would otherwise nourish the body. Additionally, tumors can release substances called cytokines that alter metabolism and promote muscle and fat breakdown.
Digestive enzyme insufficiency often accompanies pancreatic cancer when tumors block pancreatic ducts, preventing digestive enzymes from reaching the intestines. This disruption leads to malabsorption, where nutrients from food cannot be properly absorbed, further contributing to weight loss despite normal eating patterns.
Loss of appetite frequently accompanies pancreatic cancer, compounding weight loss problems. This decreased interest in food may result from tumor-related changes in metabolism, digestive difficulties causing discomfort after eating, or tumor pressure on the stomach creating early feelings of fullness.
When weight loss occurs alongside other symptoms discussed below, particularly in individuals with risk factors like long-standing diabetes, smoking history, chronic pancreatitis, or family history of pancreatic cancer, medical evaluation becomes particularly important.
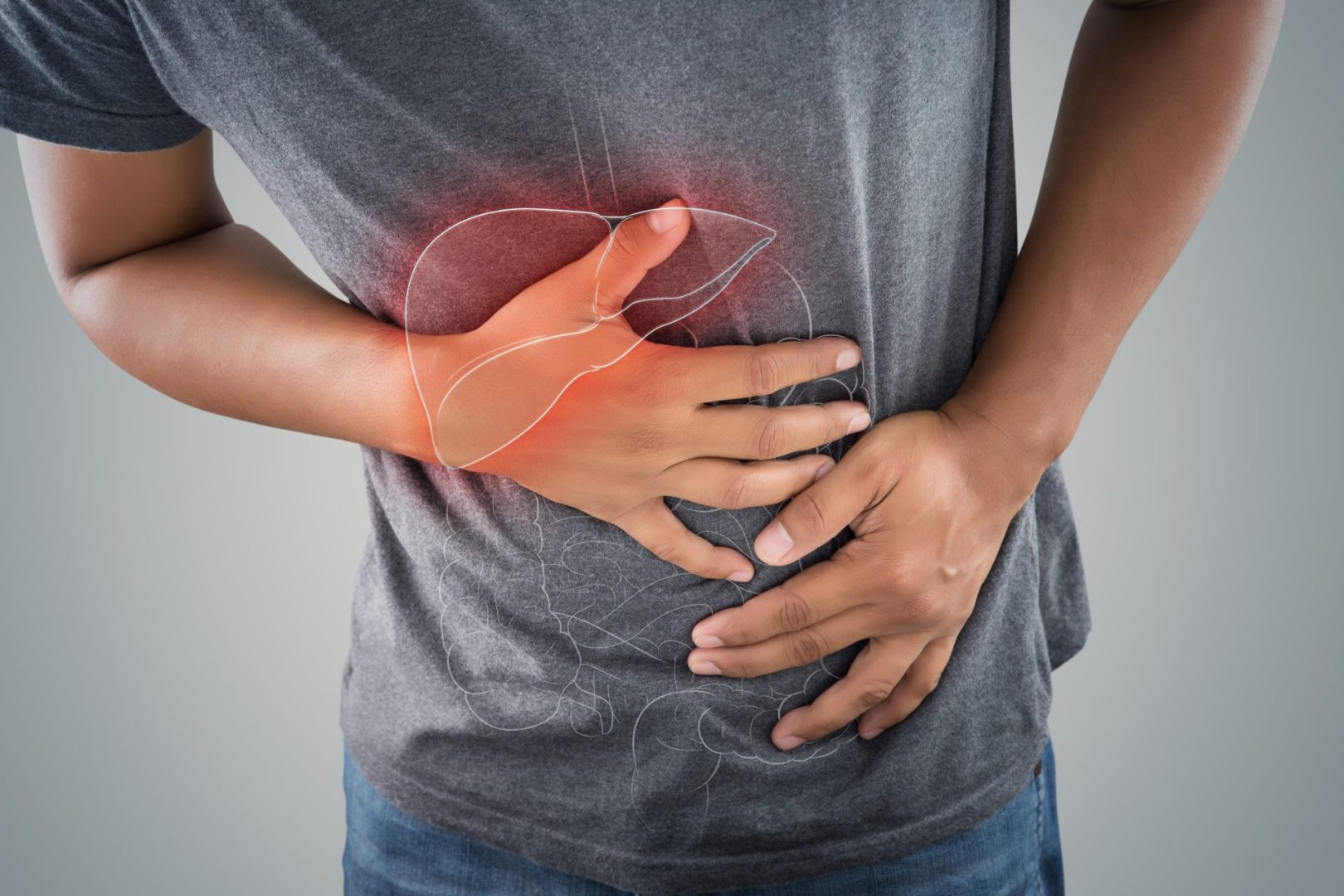
Abdominal and back pain: Characteristic discomfort patterns
Pain represents another common symptom of pancreatic cancer, with distinctive patterns that potentially help distinguish it from other gastrointestinal conditions.
The location of pancreatic cancer pain typically centers in the upper abdomen, often radiating straight through to the middle of the back. This “boring” pain that penetrates from front to back differs from many other abdominal conditions and occurs because the pancreas lies against the posterior abdominal wall near the spine.
Pain characteristics often include persistence that doesn’t resolve with position changes or over-the-counter medications. Many patients describe the discomfort as worse when lying down and somewhat improved when sitting forward, which relieves some pressure on the pancreas.
The timing of pain provides another important clue. Pain that worsens after meals suggests the tumor may be obstructing digestive enzyme flow, while pain that awakens someone from sleep (particularly between 11 PM and 3 AM) warrants special attention as a potential pancreatic cancer indicator.
Pain progression typically follows a pattern where initial mild, intermittent discomfort gradually becomes more constant and severe as the tumor grows and invades surrounding tissues and nerves. This progression distinguishes it from conditions like pancreatitis, which often causes severe pain immediately.
For cancers in the pancreatic head (the widest part closest to the small intestine), pain may develop earlier as tumors potentially compress the common bile duct, causing additional symptoms like jaundice. Cancers in the pancreatic body or tail might grow larger before causing significant pain, as they have fewer nearby structures to compress.
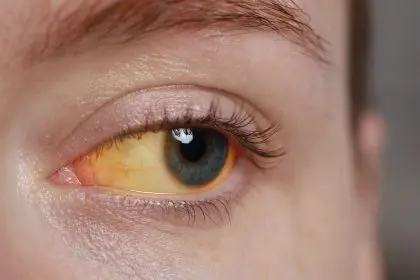
Jaundice: The most visible warning sign
Jaundice—yellowing of the skin and eyes caused by bilirubin buildup in the body—represents the most visibly apparent sign of pancreatic cancer, particularly for tumors in the head of the pancreas near the bile duct.
This distinctive yellowing typically begins subtly in the whites of the eyes (scleral icterus) before progressing to the skin. The yellowing may appear first in natural skin folds like the palms, soles, or beneath the tongue before becoming more generalized.
Accompanying changes often include darkened urine that may appear brown or tea-colored due to bilirubin excretion through the kidneys when normal bile flow becomes obstructed. This change in urine color frequently precedes noticeable skin yellowing.
Light-colored or clay-colored stools develop when bile cannot reach the intestines, as bile gives stool its characteristic brown color. This change, especially when occurring alongside darkened urine, strongly suggests bile duct obstruction requiring prompt medical evaluation.
Intense itching (pruritus) frequently accompanies jaundice as bilirubin deposits in the skin irritate nerve endings. This itching often appears worse on the palms and soles and may precede visible jaundice by days or weeks, making unexplained, intense itching a potential early warning sign.
Painless jaundice—yellowing without abdominal pain—classically suggests pancreatic cancer rather than gallstones, which typically cause painful jaundice. However, many pancreatic cancer patients do experience pain alongside jaundice, making this distinction less reliable than previously thought.
For pancreatic tumors, jaundice typically indicates a tumor in the pancreatic head compressing the common bile duct where it passes through this portion of the pancreas. While this creates an earlier warning sign than tumors in other parts of the pancreas, it also indicates the tumor’s proximity to critical structures that may complicate surgical options.
Digestive changes: Subtle but significant indicators
Various digestive disturbances frequently occur with pancreatic cancer as the tumor interferes with the organ’s essential digestive functions.
Steatorrhea—pale, foul-smelling, floating stools that may be difficult to flush—results from fat malabsorption when pancreatic enzyme production becomes impaired. These stools contain undigested fat, appear greasy or oily, and may leave an oily film on the toilet water, making them distinctly different from normal bowel movements.
New-onset digestive issues like bloating, gas, or indigestion that persist despite dietary changes or over-the-counter remedies warrant attention, particularly when they appear alongside other warning signs. While these symptoms commonly occur with numerous gastrointestinal conditions, their sudden appearance and persistence raise concern.
Changes in bowel habits, including alternating diarrhea and constipation without clear dietary or medication causes, may indicate a pancreatic tumor affecting digestive processes or pressing against the intestines. These changes typically don’t follow patterns seen with common irritable bowel syndrome.
Nausea and vomiting sometimes develop, particularly after meals, as the tumor presses against the stomach or small intestine, potentially creating partial blockages. These symptoms tend to worsen after eating fatty foods, which require more pancreatic enzyme activity for proper digestion.
Early satiety—feeling unusually full after eating small amounts—occurs when the tumor either presses directly on the stomach or affects hormonal signals regulating appetite and digestion. This symptom contributes significantly to the weight loss commonly seen with pancreatic cancer.
Blood sugar abnormalities: Metabolic warning signs
The pancreas plays a crucial role in blood sugar regulation, making new-onset diabetes or unexplained changes in existing diabetes potential warning signs of pancreatic cancer.
New-onset diabetes without typical risk factors like obesity or family history deserves particular attention, especially in individuals over 50. Research indicates that new-onset diabetes may precede other pancreatic cancer symptoms by months or even years in some cases, though most new diabetes cases do not indicate cancer.
Sudden worsening of previously well-controlled diabetes—requiring significantly more medication or insulin without clear reasons like dietary changes, weight gain, or medication interactions—may signal pancreatic involvement potentially related to cancer.
Blood sugar fluctuations that seem unusual or difficult to explain, particularly unexpected hypoglycemic (low blood sugar) episodes in someone with diabetes, might indicate pancreatic dysfunction affecting normal hormone balance between insulin and glucagon.
The relationship between diabetes and pancreatic cancer appears bidirectional. While long-standing diabetes slightly increases pancreatic cancer risk, new-onset diabetes can also result from early cancer affecting pancreatic function. This complex relationship makes monitoring changes in blood sugar patterns particularly important.
For individuals with new-onset diabetes, certain factors increase concern for possible underlying pancreatic cancer: age over 50, weight loss despite high blood sugar (which typically causes weight gain), absence of other diabetes risk factors, and presence of any other symptoms discussed in this article.
Psychological and systemic changes
Beyond physical symptoms directly related to the pancreas, pancreatic cancer often causes broader systemic effects that impact overall wellbeing.
Fatigue that seems disproportionate to activity levels and doesn’t improve with rest represents a common early complaint. This exhaustion stems from multiple factors: the body’s inflammatory response to the cancer, nutritional deficiencies from digestive impairment, and the cancer’s direct energy consumption.
Depression or anxiety sometimes precedes other pancreatic cancer symptoms, with some research suggesting this might result from tumor-produced substances affecting brain chemistry rather than simply being psychological responses to diagnosis. New-onset depression in older adults without previous history or clear precipitating factors warrants medical attention.
Unexplained fever without signs of infection occasionally occurs as the body responds to the tumor. These fevers typically run low-grade (below 100.4°F or 38°C) and may come and go, often accompanying other systemic symptoms like night sweats or fatigue.
Blood clots forming without obvious cause (idiopathic thromboembolism) have a well-established association with pancreatic cancer due to cancer cells releasing substances that increase blood coagulation. Unexplained blood clots, particularly in unusual locations or recurring despite treatment, sometimes represent the first detectable sign of pancreatic cancer.
Unintentional weight loss combined with muscle wasting disproportionate to reduced food intake may indicate cachexia—a complex metabolic syndrome associated with advanced cancer. This condition differs from simple weight loss, as it involves inflammatory processes causing muscle breakdown that dietary changes alone cannot reverse.
Risk factors that elevate concern for warning signs
Certain risk factors increase the likelihood that the symptoms described above might indicate pancreatic cancer, making prompt medical evaluation particularly important for these individuals.
Age represents a significant risk factor, with most pancreatic cancers diagnosed after age 65 and the risk increasing with each decade. When the previously described symptoms appear in older adults, especially those over 60, the possibility of pancreatic cancer increases substantially.
Smoking history dramatically raises pancreatic cancer risk, with current smokers facing approximately twice the risk of never-smokers. The risk remains elevated for former smokers but decreases gradually over time after quitting. Symptoms in individuals with significant smoking history warrant thorough evaluation.
Family history significantly impacts risk assessment, particularly having two or more first-degree relatives (parents, siblings, children) with pancreatic cancer or one first-degree relative diagnosed before age 50. Certain genetic syndromes like BRCA1/2 mutations, Lynch syndrome, and Peutz-Jeghers syndrome also increase risk.
Chronic pancreatitis, particularly long-standing inflammation lasting more than five years, creates an environment where pancreatic cancer risk increases substantially. Symptoms that change from typical pancreatitis patterns deserve careful assessment to distinguish between chronic inflammation and potential malignancy.
Newly diagnosed diabetes in the absence of typical risk factors, particularly in individuals over 50 experiencing unexplained weight loss, creates a scenario where pancreatic cancer screening may be warranted, especially with additional symptoms.
African American ethnicity carries increased pancreatic cancer risk compared to other racial groups in the United States, with African Americans having a 50-90% higher incidence rate than white Americans. This elevated risk makes symptom evaluation particularly important in this population.
Diagnostic pathways when symptoms appear
When warning signs suggest possible pancreatic cancer, several diagnostic approaches help confirm or rule out this possibility.
Initial evaluation typically includes comprehensive blood tests examining liver function, pancreatic enzymes, tumor markers (particularly CA 19-9, though it has limitations), blood counts, and nutritional status. These tests provide important baseline information but cannot definitively diagnose or exclude pancreatic cancer.
Imaging studies form the cornerstone of pancreatic cancer detection. Contrast-enhanced CT scans represent the most common initial imaging, potentially identifying tumors, duct abnormalities, and whether the cancer has spread beyond the pancreas. MRI provides additional detail, particularly for smaller lesions, while endoscopic ultrasound offers the highest sensitivity for small tumors by placing the ultrasound probe directly adjacent to the pancreas through the stomach wall.
Endoscopic retrograde cholangiopancreatography (ERCP) allows visualization and sampling of the pancreatic and bile ducts while potentially relieving biliary obstruction causing jaundice. This procedure combines endoscopy with contrast dye to examine the ducts in detail and can collect cell samples for analysis.
Tissue diagnosis ultimately provides definitive confirmation of pancreatic cancer. Samples may be obtained through fine needle aspiration during endoscopic ultrasound, CT-guided biopsy, or during ERCP. In some cases, particularly when imaging strongly suggests pancreatic cancer and immediate surgery is planned, biopsy might be deferred until surgery.
Genetic testing increasingly plays a role in pancreatic cancer management, potentially identifying inherited mutations that influenced cancer development and might impact treatment decisions. This testing has particular importance for families with multiple affected members or early-onset cases.
Taking action: When to seek medical evaluation
The diffuse nature of pancreatic cancer symptoms creates challenges in knowing when to seek medical attention. Several guidelines can help make this determination.
Persistent symptoms lasting more than two weeks without obvious explanation warrant medical evaluation, particularly when they include jaundice, unexplained weight loss, new-onset diabetes with weight loss, or the characteristic abdominal pain radiating to the back.
Symptom combinations dramatically increase concern. While individual symptoms like occasional indigestion rarely indicate pancreatic cancer, combinations such as weight loss with abdominal pain, new diabetes with unexplained digestive changes, or fatigue with early satiety raise the level of concern substantially.
Age considerations affect decision-making, with identical symptoms causing greater concern in older adults than younger individuals. The substantial increase in pancreatic cancer incidence after age 50 makes prompt evaluation of new digestive or systemic symptoms particularly important in this age group.
Personal risk factor assessment should influence how quickly you seek medical attention for concerning symptoms. Individuals with multiple risk factors like smoking history, chronic pancreatitis, family history of pancreatic or related cancers, or certain genetic syndromes should have a lower threshold for seeking evaluation.
Clear communication with healthcare providers about the full constellation of symptoms proves crucial for proper assessment. When seeking medical evaluation, prepare a timeline noting when each symptom began, how it has changed over time, and any factors that seem to worsen or improve it. This detailed information helps physicians recognize patterns suggestive of pancreatic disease.
While pancreatic cancer’s reputation as a “silent disease” has some validity, recognizing these warning signs—particularly when they occur in combination or with relevant risk factors—creates opportunities for earlier diagnosis when treatment offers the greatest benefit. Understanding these signals empowers individuals to advocate for appropriate evaluation when concerning patterns emerge.