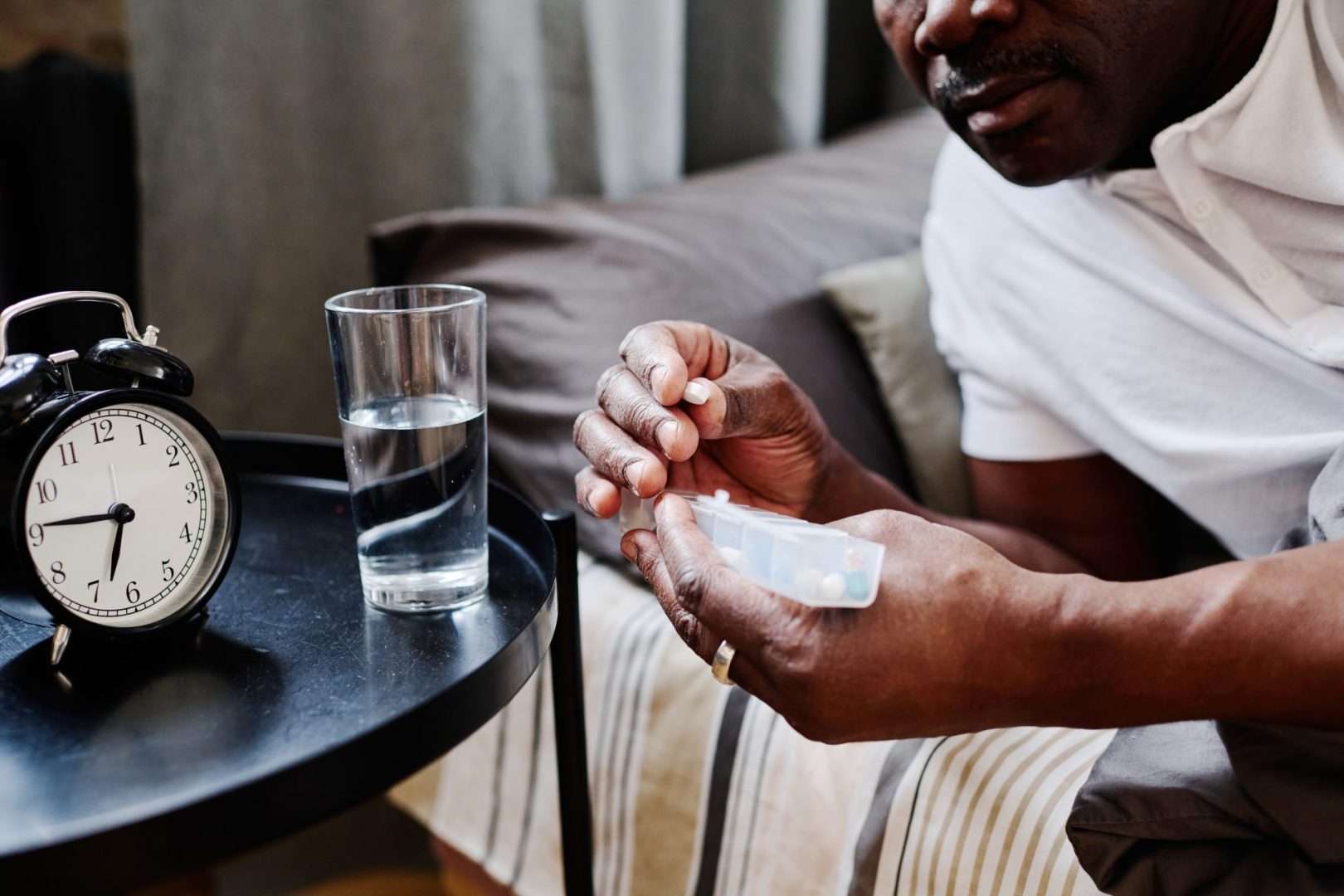
For the millions of people worldwide living with dementia, medication management presents unique challenges. While certain drugs help manage symptoms and slow cognitive decline, others can dramatically worsen confusion, memory problems, and overall functioning. Healthcare providers face the delicate balance of treating concurrent conditions without exacerbating cognitive impairment. This comprehensive guide examines medications that are generally unsuitable for people with dementia, explaining why they pose risks and what alternatives might offer safer options.
Anticholinergic medications
Anticholinergic drugs block acetylcholine, a crucial neurotransmitter for memory and learning—the very functions already compromised in dementia. These medications can significantly worsen cognitive function, cause confusion, and accelerate decline in people with dementia. The anticholinergic effect occurs with varying potency across multiple drug classes.
First-generation antihistamines like diphenhydramine (found in many over-the-counter sleep aids and allergy medications) exhibit strong anticholinergic properties. These medications easily cross the blood-brain barrier, potentially causing confusion, blurred vision, constipation, and urinary retention. The cognitive effects can mimic worsening dementia, leading to misdiagnosis of disease progression rather than medication side effects.
Certain bladder medications for incontinence, including oxybutynin and tolterodine, work specifically by blocking acetylcholine receptors. These medications treat overactive bladder but can dramatically impair cognition in people with dementia. Studies indicate that even short-term use can lead to noticeable cognitive decline, which may persist for weeks after discontinuation.
Older antidepressants, particularly tricyclic antidepressants such as amitriptyline, imipramine, and doxepin, have significant anticholinergic effects. While these medications effectively treat depression, their cognitive side effects make them unsuitable for people with dementia. The cognitive impact can include confusion, memory problems, and disorientation beyond what the underlying dementia causes.
The anticholinergic burden becomes especially problematic when multiple medications with these properties are prescribed simultaneously, creating a cumulative effect that dramatically increases cognitive impairment risk. Medication reviews using standardized scales like the Anticholinergic Cognitive Burden Scale can help identify and reduce this cumulative burden.
Benzodiazepines and sedative-hypnotics
Benzodiazepines like diazepam, lorazepam, and alprazolam are commonly prescribed for anxiety and insomnia but pose particular risks for people with dementia. These medications can cause excessive sedation, confusion, impaired balance leading to falls, and paradoxical agitation in older adults with cognitive impairment.
The cognitive effects of benzodiazepines often mimic dementia itself, sometimes leading to what’s called “pseudodementia,” where medication side effects are mistaken for disease progression. These drugs affect short-term memory formation and information processing—cognitive functions already compromised in dementia—creating a compounding effect on impairment.
Long-acting benzodiazepines present even greater risks due to their extended half-life in older adults. Medications like diazepam can accumulate in the body over time, leading to prolonged cognitive impairment and increased fall risk. The body’s ability to metabolize these medications decreases with age, extending their effects beyond what might be expected from standard dosing.
The “Z-drugs” (non-benzodiazepine sedative-hypnotics) including zolpidem, zaleplon, and eszopiclone were once thought to be safer alternatives but now show similar risk profiles in people with dementia. While they may have shorter half-lives, their effects on cognition, balance, and fall risk remain significant concerns, particularly when used beyond recommended short-term treatment periods.
Withdrawal symptoms from benzodiazepines can be especially problematic for people with dementia who may not understand why they’re experiencing increased anxiety, insomnia, or agitation. This makes discontinuation challenging and necessitates careful tapering rather than abrupt cessation.
Antipsychotic medications
Antipsychotic medications present a particularly complicated situation for dementia care. While sometimes necessary for managing severe behavioral symptoms, these medications come with substantial risks that often outweigh their benefits for most people with dementia.
Conventional (typical) antipsychotics like haloperidol have long been known to cause serious movement disorders, including parkinsonian symptoms, tardive dyskinesia, and akathisia. These medications also significantly increase sedation and cognitive dulling, often worsening the very symptoms they’re prescribed to address.
Atypical antipsychotics (second-generation antipsychotics) including risperidone, olanzapine, and quetiapine carry black box warnings from regulatory agencies regarding increased mortality risk when used in older adults with dementia. Studies indicate approximately a 1.7 times higher risk of death, primarily from cardiovascular events and infections, compared to placebo.
The cognitive effects of antipsychotics in people with dementia include increased confusion, sedation, and reduced awareness of surroundings. These medications can make communication more difficult and decrease quality of life through oversedation. They may induce a “chemical restraint” effect that reduces behavioral symptoms by broadly suppressing brain activity rather than addressing underlying causes.
Long-term use of antipsychotics in dementia care has been associated with faster cognitive decline, increased risk of pneumonia, accelerated mortality, and reduced quality of life. Despite these risks, antipsychotic prescribing remains common in residential care settings, often continuing beyond recommended time frames for reassessment and potential discontinuation.
Strong opioid pain medications
Pain management presents unique challenges in dementia care, as unaddressed pain often manifests as behavioral disturbances. However, strong opioids can worsen confusion, increase fall risk, and create additional complications for people with cognitive impairment.
Medications like oxycodone, hydromorphone, and fentanyl have heightened effects in older adults due to age-related changes in drug metabolism and elimination. The sedative properties of these medications can compound cognitive impairment and increase the risk of respiratory depression, particularly in frail older adults with dementia.
Opioid side effects often mirror or exacerbate dementia symptoms, including confusion, hallucinations, and changes in mood. This overlap makes it difficult to distinguish medication effects from disease progression, potentially leading to inappropriate medication adjustments or missed adverse reactions.
The constipation caused by opioids presents particular challenges for people with dementia who may not be able to communicate discomfort effectively. Severe constipation can lead to fecal impaction, intestinal obstruction, or overflow incontinence—conditions that significantly impact quality of life and can precipitate delirium episodes.
Opioid use in dementia requires exceptionally careful monitoring, starting with the lowest effective dose and regular reassessment. When necessary, the structured approach of “start low, go slow, and regularly review” provides the safest framework for opioid use in this vulnerable population.
Medications with strong anticholinergic properties
Certain classes of medications have pronounced anticholinergic effects, making them particularly problematic for people with dementia. Antispasmodics like dicyclomine and hyoscyamine, used for gastrointestinal conditions, can dramatically worsen cognitive function while treating relatively minor symptoms.
Skeletal muscle relaxants including cyclobenzaprine and orphenadrine combine anticholinergic properties with central nervous system depression, creating dual mechanisms for cognitive impairment. These medications often provide minimal benefit for chronic pain conditions while introducing substantial cognitive risks.
Certain antiparkinson medications, particularly trihexyphenidyl and benztropine, have strong anticholinergic properties that can worsen cognitive function in people with Parkinson’s disease who also have dementia. The balance between treating motor symptoms and preserving cognitive function requires careful medication selection and monitoring.
Over-the-counter sleep aids containing diphenhydramine or doxylamine present particular risks since they’re easily accessible without medical supervision. People with dementia or their caregivers may not realize these seemingly benign sleep aids can significantly worsen confusion and cognitive function.
The effects of these medications can persist for days or weeks after discontinuation in older adults due to their prolonged elimination half-life and potential accumulation in fatty tissues. This extended effect period means cognitive impairment may continue long after the medication has been stopped.
Medications with orthostatic hypotension risks
Medications that lower blood pressure, particularly when changing positions, pose special risks for people with dementia who already have increased fall risk and potential gait instability. Several medication classes contribute significantly to this risk.
Alpha-blockers like doxazosin, prazosin, and tamsulosin, commonly prescribed for prostate enlargement or hypertension, can cause significant drops in blood pressure when standing. This orthostatic hypotension increases fall risk and can reduce cerebral perfusion, temporarily worsening cognitive function.
Antihypertensive medications, while often necessary, require careful monitoring in people with dementia. Medications like clonidine, methyldopa, and hydralazine have higher risks of central nervous system side effects and orthostatic hypotension compared to alternatives like ACE inhibitors or calcium channel blockers.
Diuretics, particularly loop diuretics like furosemide, can cause rapid fluid shifts leading to electrolyte imbalances and dehydration. These changes can precipitate confusion, falls, and acute kidney injury in people with dementia, especially those with inconsistent fluid intake or difficulty communicating thirst.
Vasodilators including nitrates and minoxidil can cause dramatic blood pressure fluctuations, particularly problematic for people with dementia who may not recognize warning symptoms of hypotension like lightheadedness or visual changes before falls occur.
The combination of multiple blood pressure-lowering medications dramatically increases orthostatic hypotension risk beyond what might be expected from individual medications. This “polypharmacy effect” necessitates regular medication reviews with attention to standing blood pressure measurements in people with dementia.
Medications affecting glucose levels
Blood glucose fluctuations can significantly impact cognitive function and behavior in people with dementia, making medications that alter glucose levels particularly concerning. Certain diabetes medications require special consideration in this population.
Sulfonylureas like glipizide, glyburide, and glimepiride stimulate insulin release independent of blood glucose levels, creating significant hypoglycemia risk. Hypoglycemia can cause confusion, agitation, dizziness, weakness, and even seizures—symptoms that may be attributed to dementia rather than recognized as medication effects.
Insulin therapy, while sometimes necessary, carries substantial hypoglycemia risk if dosing doesn’t account for changing eating patterns common in dementia. As dementia progresses, meal consumption often becomes irregular or decreased, making fixed insulin dosing regimens increasingly problematic.
The cognitive effects of hypoglycemia can persist even after blood glucose normalizes, a phenomenon called “post-hypoglycemic cognitive dysfunction.” This extended effect means even brief hypoglycemic episodes can impact cognitive function for days afterward in people with dementia.
The symptoms of hypoglycemia—confusion, irritability, diaphoresis, shakiness—may not be recognized or reported by people with dementia, delaying treatment and allowing more severe symptoms to develop. This communication barrier makes hypoglycemia particularly dangerous in this population.
Diabetes management goals typically shift toward preventing symptomatic hyperglycemia and avoiding hypoglycemia rather than tight glycemic control as dementia progresses. This approach acknowledges the changing risk-benefit balance of intensive glucose management in people with cognitive impairment.
Medications with high fall risk profiles
Falls represent a leading cause of injury, hospitalization, and death among people with dementia. Medications that increase fall risk through multiple mechanisms require careful consideration in this already vulnerable population.
Sedating antihistamines found in many over-the-counter combination cold, allergy, and sleep products cause drowsiness and impaired coordination beyond their anticholinergic effects. These medications often have prolonged effects in older adults due to decreased metabolism and elimination.
Certain antidepressants, particularly mirtazapine and trazodone, can cause significant morning drowsiness and orthostatic hypotension even when prescribed at low doses for insomnia. These effects can persist well into the following day, creating extended periods of fall risk.
Medications with alpha-blocking properties, including some antipsychotics and antihypertensives, create postural instability through sudden blood pressure drops when changing positions. This effect combines with the coordination and balance problems already present in many people with dementia.
The sedating effects of multiple medications with central nervous system effects often produce multiplicative rather than simply additive effects on fall risk. This synergistic effect means that combinations of medications at seemingly appropriate individual doses can create dramatic increases in fall risk.
Environmental factors interact with medication effects to further increase fall risk. Medications causing nocturia (nighttime urination), including diuretics and some bladder medications, create particularly high-risk situations when combined with the disorientation and poor environmental awareness common in dementia.
Medications with delirium risk
Delirium—an acute confusional state characterized by fluctuating attention and awareness—occurs more frequently in people with dementia and can accelerate cognitive decline. Certain medications dramatically increase delirium risk in this vulnerable population.
Anticholinesterase inhibitors with strong peripheral effects, including certain insecticides and some medications used for myasthenia gravis, can precipitate central cholinergic toxicity with features of delirium. This reaction appears counterintuitive since cholinesterase inhibitors are used to treat dementia, but excessive cholinergic stimulation produces paradoxical effects.
Some antibiotics, particularly fluoroquinolones like ciprofloxacin and levofloxacin, have neurotoxic effects that can trigger delirium, especially when kidney function is impaired. These antibiotics cross the blood-brain barrier effectively and can directly impact neurotransmitter systems already compromised in dementia.
Anti-seizure medications, particularly older generations like phenobarbital and phenytoin, have significant cognitive side effects that can precipitate delirium in people with dementia. Even therapeutic blood levels can produce toxic central nervous system effects in this sensitive population.
The use of bladder antimuscarinic drugs for incontinence combines multiple delirium risk factors: anticholinergic effects, blood-brain barrier penetration, and disruption of sleep through incomplete bladder emptying. This combination makes medications like oxybutynin particularly high-risk for precipitating delirium.
Delirium episodes triggered by medications often lead to hospitalization, which itself increases the risk of functional decline, further cognitive impairment, and mortality in people with dementia. This cascade effect makes medication-induced delirium prevention particularly important.
Safer prescribing approaches
Safer medication management for people with dementia involves several key principles that balance symptom management with cognitive protection. These approaches help minimize medication-related harm while addressing necessary medical conditions.
Medication reviews should occur regularly, ideally every 3-6 months, with special attention to anticholinergic burden, sedative load, and medications on inappropriate prescribing lists. These structured reviews identify opportunities for deprescribing—the systematic process of identifying and discontinuing medications where harm outweighs benefit.
Non-pharmacological approaches should be considered first-line for many symptoms. Behavioral interventions for sleep disturbances, environmental modifications for anxiety, and structured activities for agitation often provide better outcomes without medication-related cognitive impairment.
The concept of “start low, go slow, but go” guides appropriate prescribing when medications are necessary. Starting at lower doses than standard recommendations, titrating gradually, but reaching therapeutic doses when needed helps balance safety with symptom management.
Medication simplification through once-daily dosing, minimizing “as needed” medications that may be inconsistently administered, and reducing overall medication number improves adherence and reduces adverse effects. This simplification becomes increasingly important as dementia progresses.
The involvement of both caregivers and people with dementia in medication decisions whenever possible improves adherence and ensures treatments align with goals of care. This shared decision-making approach acknowledges the changing balance between symptom management and quality of life as dementia progresses.
Managing medications appropriately for people with dementia requires careful consideration of cognitive impact alongside other treatment goals. By recognizing high-risk medications and implementing safer prescribing practices, healthcare providers can help preserve cognitive function while addressing essential health needs. Regular medication reviews, consideration of non-drug alternatives, and patient-centered decision-making create a framework for optimizing health outcomes in this vulnerable population.