Blood clotting represents one of the body’s most elegant and complex protective mechanisms. This intricate process involves numerous proteins working in precise sequence – often described as a clotting cascade – to prevent excessive bleeding after injury. Under normal circumstances, when a blood vessel sustains damage, platelets rush to the site and form a temporary plug while clotting factors activate in a specific order to create a more permanent seal.
Hemophilia A disrupts this vital process by specifically affecting Factor VIII (8), a crucial component in the clotting sequence. Without sufficient quantities of functioning Factor VIII, the cascade falters at a critical juncture, preventing proper clot formation and allowing bleeding to continue longer than it should. This fundamental disruption explains the characteristic symptoms and complications associated with the disorder.
Understanding this mechanism helps explain why seemingly minor injuries can produce disproportionate bleeding responses in those with hemophilia A. What might cause minimal bleeding in someone without the condition can become a serious health concern requiring immediate intervention for someone with this disorder.
Recognizing hemophilia A warning signs
Hemophilia A manifests through several characteristic symptoms that vary in severity depending on Factor VIII levels in the bloodstream. Even with the same genetic alteration, symptom presentation may differ significantly between individuals.
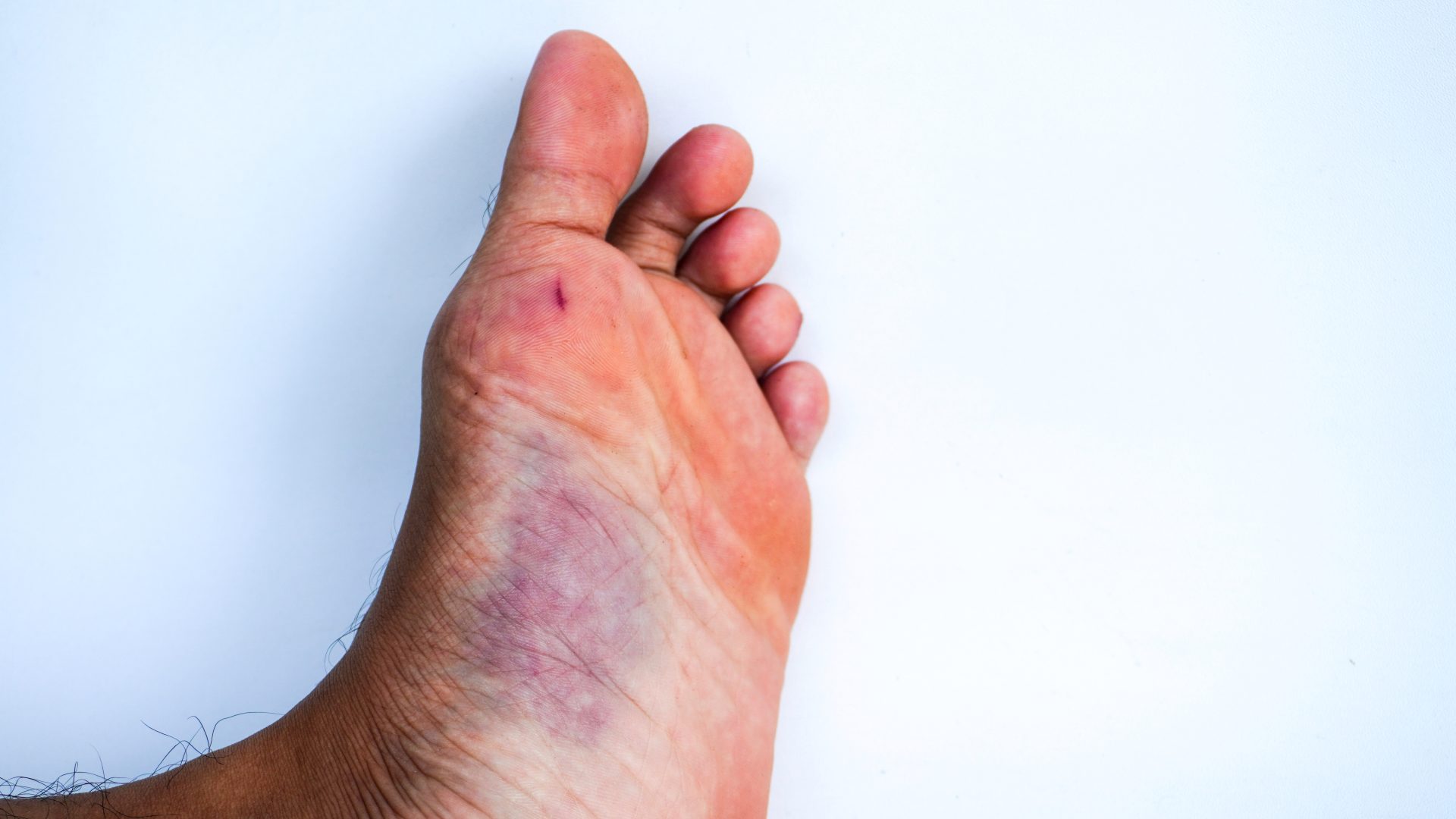
Easy bruising often represents the most visible and earliest sign of hemophilia A. These bruises typically appear larger than expected for the injury that caused them and may develop after minimal trauma or even spontaneously. Parents might notice this tendency in young children before other symptoms become apparent.
Prolonged bleeding after injuries or medical procedures provides another key indicator. Cuts or scrapes that continue bleeding beyond normal timeframes, excessive bleeding after dental work, or unexpectedly heavy bleeding during surgical procedures all suggest potential clotting disorders that warrant further investigation.
Joint bleeding represents one of the most problematic and painful manifestations of hemophilia A. These hemorrhages typically affect weight-bearing joints including knees, ankles, and elbows. The blood accumulating within the joint space causes swelling, heat, pain, and restricted movement. Without proper treatment, repeated episodes can lead to chronic joint damage over time.
Internal bleeding constitutes the most serious complication, occurring in muscles, organs, or the brain. These internal hemorrhages may present with symptoms including pain, swelling, headaches, confusion, or loss of consciousness depending on their location. Such bleeding events require immediate medical attention as they can become life-threatening.
Importantly, symptom severity correlates with the amount of functioning Factor VIII present. Mild hemophilia (5-40% of normal Factor VIII) may only cause excessive bleeding after significant injuries or surgery. Moderate hemophilia (1-5% of normal Factor VIII) typically produces excessive bleeding after relatively minor injuries. Severe hemophilia (less than 1% of normal Factor VIII) can cause spontaneous bleeding with no apparent trigger.
The genetic patterns behind hemophilia A
Hemophilia A follows an X-linked recessive inheritance pattern, meaning the altered gene resides on the X chromosome. This genetic arrangement explains several characteristic patterns observed in families affected by the condition.
Males, having only one X chromosome, develop hemophilia A when they inherit an affected X chromosome from their mother. With no second X chromosome to provide a functioning copy of the gene, they always express the condition if they carry the altered gene.
Females typically serve as carriers rather than developing the full condition because they possess two X chromosomes. Even with one affected X chromosome, the normal gene on their other X chromosome usually produces enough Factor VIII to prevent significant symptoms. However, some female carriers experience mild bleeding symptoms due to a phenomenon called lyonization, where one X chromosome becomes partially inactivated.
Family history often provides important clues for diagnosis, though approximately 30% of hemophilia A cases arise from spontaneous genetic alterations with no previous family history. This spontaneous mutation pattern explains why hemophilia A can appear unexpectedly in families with no known bleeding disorders.
Genetic testing now allows precise identification of the specific Factor VIII gene alteration responsible for an individual’s hemophilia A. This information helps confirm diagnoses, identify carriers, enable family planning decisions, and sometimes predict disease severity based on how the genetic change affects Factor VIII production and function.
5 treatment approaches revolutionizing hemophilia care
- Factor replacement therapy remains the cornerstone of hemophilia A treatment. This approach involves infusing purified Factor VIII concentrates to temporarily restore clotting ability. Modern products derive from either purified human plasma or recombinant DNA technology, with recombinant products eliminating the infection risks previously associated with plasma-derived treatments. Traditional replacement therapy requires infusions two to three times weekly for prevention or immediately after bleeding begins.
- Extended half-life products represent a significant advancement in Factor VIII therapy. These modified clotting factors remain active in the bloodstream longer, reducing infusion frequency to once weekly or less for many patients. This decreased treatment burden improves quality of life while maintaining protection against bleeding episodes.
- Non-factor replacement therapies operate through alternative mechanisms that bypass the need for Factor VIII completely. These innovative treatments work by mimicking Factor VIII’s role in the clotting cascade or by reducing the activity of natural anticoagulants. Such approaches prove particularly valuable for patients who have developed inhibitors (antibodies against Factor VIII) that render traditional replacement ineffective.
- Gene therapy holds tremendous promise for potentially curing hemophilia A rather than simply managing symptoms. This approach introduces functioning copies of the Factor VIII gene into the patient’s cells, enabling their body to produce the clotting factor independently. Early clinical trials show encouraging results with some participants maintaining near-normal Factor VIII levels for several years after a single treatment. Though still experimental, gene therapy represents the frontier of hemophilia research.
- Home therapy programs enable patients or caregivers to administer treatments outside medical settings. This approach allows immediate response to bleeding episodes and facilitates regular preventive infusions. Modern infusion devices, simplified administration techniques, and comprehensive training programs make home therapy increasingly accessible, dramatically improving quality of life while reducing hospitalization rates.
Prophylactic treatment schedules, which involve regular factor infusions to prevent bleeding before it occurs, have become the standard of care for severe hemophilia A. This preventive approach has transformed management from reactive emergency care to proactive health maintenance, significantly reducing joint damage and improving long-term outcomes.
Living well with hemophilia A
Beyond medical treatments, several lifestyle approaches help manage hemophilia A effectively. These strategies complement medical interventions to reduce bleeding risk and minimize complications.
Physical activity, when properly selected and supervised, provides tremendous benefits rather than dangers. Low-impact exercises like swimming, cycling, and walking strengthen muscles without stressing joints, while improved muscle tone helps stabilize and protect joints from bleeding episodes. Working with physical therapists who understand hemophilia helps develop appropriate exercise routines tailored to individual needs and limitations.
Joint protection strategies help prevent the chronic arthritis that historically plagued hemophilia patients. Proper body mechanics, assistive devices when needed, maintaining healthy weight, and avoiding high-impact activities all contribute to preserving joint function throughout life.
Dental hygiene takes on special importance given the bleeding risks associated with dental procedures. Regular brushing, flossing, and preventive dental care help avoid more invasive treatments that might trigger significant bleeding. When dental work becomes necessary, coordination between dental providers and hemophilia treatment centers ensures appropriate precautions.
Comprehensive care teams provide the most successful management approach. These specialized groups typically include hematologists, nurses, physical therapists, social workers, and genetic counselors working together to address all aspects of living with hemophilia A. This coordinated care model addresses not only bleeding prevention but also pain management, mental health support, educational needs, and family planning considerations.
Pregnancy and family planning considerations
For women who carry the hemophilia A gene, pregnancy and family planning involve several important considerations. Understanding these factors helps ensure informed decisions and appropriate care during pregnancy.
Genetic counseling provides valuable information about the likelihood of transmitting hemophilia A to children. For female carriers, each son has a 50% chance of having hemophilia A, while each daughter has a 50% chance of being a carrier. Several options exist for those wishing to prevent transmission, including preimplantation genetic diagnosis during in vitro fertilization.
Pregnancy itself generally proceeds normally for hemophilia carriers, though some experience increased bleeding tendency during delivery due to lower-than-average Factor VIII levels. Close coordination between obstetricians and hemophilia treatment specialists ensures appropriate monitoring and intervention if needed.
Male infants born to known carriers can be tested immediately after birth, allowing early intervention before significant bleeding episodes occur. This prompt diagnosis enables prophylactic treatment to begin when appropriate, potentially preventing joint damage and other complications.
Looking toward the future of hemophilia care
Research into hemophilia A continues advancing rapidly, with several promising directions likely to transform care in coming years. Novel approaches under investigation include longer-acting replacement products, subcutaneous administration options, and gene editing technologies that could potentially cure the condition permanently.
Access to advanced treatments remains uneven globally, with significant efforts underway to improve care in regions with limited resources. International collaborations focus on developing affordable treatment options, establishing care centers, and training healthcare providers in hemophilia management.
Early diagnosis through newborn screening programs could further improve outcomes by allowing treatment before damaging bleeding episodes occur. Though not yet universally implemented, such screening appears increasingly feasible as testing becomes more efficient and cost-effective.
With comprehensive care, modern treatments, and appropriate lifestyle modifications, most individuals with hemophilia A now enjoy near-normal life expectancy and significantly improved quality of life compared to previous generations. This dramatic progress transforms hemophilia A from a potentially devastating condition to a manageable chronic disorder with continuously improving outcomes.