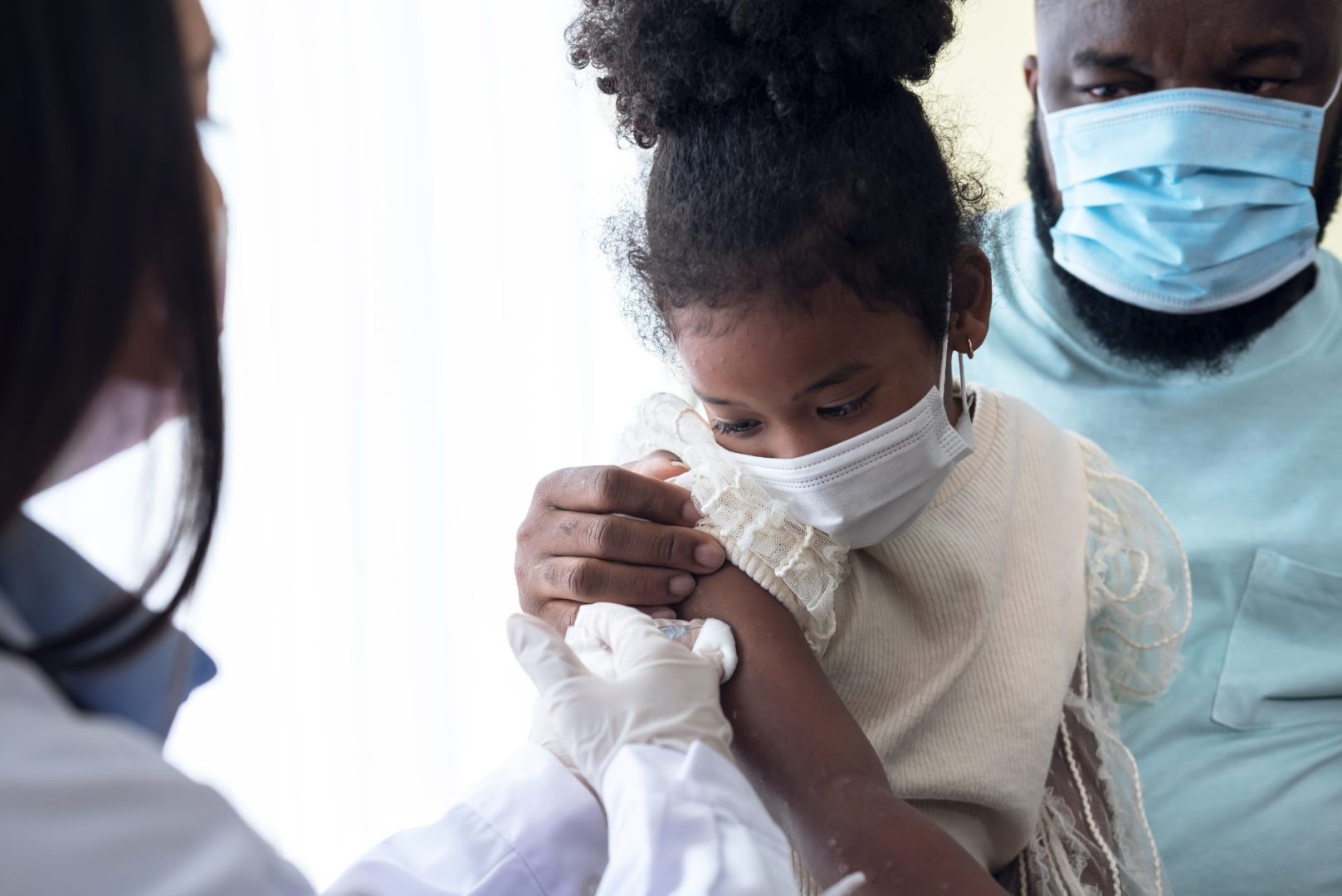
Health and Human Services Secretary Robert F. Kennedy Jr. has removed Covid-19 vaccines from the CDC’s recommended immunization schedule for healthy children and pregnant women, marking a dramatic policy shift that medical professionals warn could have serious health consequences. The announcement, made through a social media video, bypassed the standard scientific review process typically required for such significant changes to public health recommendations.
The decision represents one of the most controversial moves since Kennedy assumed his position, directly contradicting decades of established vaccine safety protocols and scientific consensus. Medical professionals across the country have expressed alarm about the potential impact on vulnerable populations, particularly pregnant women and infants who face higher risks from Covid-19 infections.
Kennedy’s announcement came without presentation of scientific evidence to support the policy change, raising questions about the decision-making process and the role of scientific expertise in public health policy. The move stands in stark contrast to his previous statements about not taking vaccines away from people and ensuring access to good science for informed decision-making.
The timing of this announcement has surprised many in the medical community, as it comes during a period when health officials were already considering more targeted, risk-based approaches to Covid-19 vaccination recommendations rather than blanket removal of recommendations for entire population groups.
The announcement that shocked medical professionals
Kennedy’s video announcement featured him alongside FDA Commissioner Dr. Marty Makary and National Institutes of Health Director Dr. Jay Bhattacharya at the Health and Human Services headquarters. The setting suggested official government backing for the policy change, though the CDC’s online immunization schedule had not been updated as of the announcement.
The health secretary specifically criticized previous administration policies, stating that healthy children had been urged to receive additional Covid shots despite what he characterized as a lack of clinical data supporting repeat booster strategies in pediatric populations. This characterization contradicts extensive research conducted on pediatric Covid vaccination safety and efficacy.
The unusual nature of the announcement, delivered through social media rather than through traditional medical or scientific channels, has raised concerns about the transparency and scientific rigor behind the decision. Medical professionals typically expect such significant policy changes to be accompanied by detailed scientific justification and peer review.
The visual presentation of the announcement, with Kennedy flanked by other health officials, appeared designed to project authority and consensus, though the lack of scientific backing has undermined the credibility of the message among medical professionals.
Medical community raises alarm about consequences
Healthcare professionals have expressed serious concerns about the potential impact of removing Covid-19 vaccine recommendations for pregnant women and healthy children. These populations face documented higher risks from Covid-19 infections, making vaccination particularly important for protecting their health.
Pregnant women experience increased risks of severe Covid-19 complications, including hospitalization and adverse pregnancy outcomes. Research has consistently shown that Covid-19 vaccination during pregnancy reduces these risks while also providing protection to newborn infants through maternal antibodies.
Infants under six months of age, who are too young to receive Covid-19 vaccines themselves, rely on maternal vaccination for protection during their most vulnerable months. Studies have documented that the majority of hospitalized infants in this age group are born to unvaccinated mothers, highlighting the importance of maternal vaccination.
The removal of recommendations could create confusion among healthcare providers about appropriate care for pregnant patients and pediatric populations. Many physicians rely on CDC guidance to inform their clinical decision-making and patient counseling.
Medical organizations have also expressed concern that the decision could undermine public confidence in other established vaccine recommendations, potentially affecting vaccination rates for other preventable diseases.
Insurance and access implications create new barriers
The policy change raises significant questions about insurance coverage and government funding for Covid-19 vaccines for the affected populations. Under current healthcare regulations, insurance companies are required to cover vaccines recommended by the CDC’s Advisory Committee on Immunization Practices.
Without formal CDC recommendations, insurance companies may no longer be required to provide coverage for Covid-19 vaccines for healthy children and pregnant women. While some insurers might choose to continue coverage based on scientific evidence supporting vaccine safety and effectiveness, there is no guarantee of consistent coverage across all plans.
The federal Vaccines for Children program, which provides vaccines to children who cannot otherwise afford them, typically covers vaccines recommended by the CDC. The removal of recommendations could eliminate this coverage option, creating access barriers for low-income families.
Healthcare providers may face challenges in obtaining vaccines for these populations if government purchasing programs no longer include them. This could create supply issues and increase costs for medical practices trying to serve vulnerable patients.
The potential legal ramifications of the decision could lead to court challenges, as medical professionals and advocacy groups may argue that the removal of recommendations without scientific justification violates public health standards.
Bypassing established scientific review processes
Kennedy’s announcement circumvented the normal process for evaluating and changing vaccine recommendations, which typically involves extensive scientific review and input from independent medical experts. This established process ensures that policy changes are based on rigorous analysis of safety and efficacy data.
The standard procedure involves FDA evaluation of clinical trial data and other research to assess vaccine safety and effectiveness. Following FDA approval, the CDC and its Advisory Committee on Immunization Practices review the evidence and make recommendations about appropriate use, including target populations and timing.
The Advisory Committee on Immunization Practices consists of independent medical experts who volunteer their time to review scientific evidence and provide recommendations based on their expertise. This committee structure is designed to ensure that vaccine recommendations reflect current scientific understanding rather than political considerations.
By bypassing this established process, Kennedy’s decision has raised concerns about the role of scientific expertise in public health policy and the potential for political considerations to override medical evidence in future decisions.
The departure from normal procedures has also created uncertainty about the legal and regulatory status of the decision, as it unclear whether the change has been formally implemented through appropriate channels.
Previous policy considerations and ongoing debates
Before Kennedy’s announcement, the CDC’s vaccine advisory committee had been considering modifications to Covid-19 vaccine recommendations, but these discussions focused on more targeted, risk-based approaches rather than complete removal of recommendations for healthy populations.
The committee had been weighing whether to move away from blanket recommendations for everyone six months and older to more specific recommendations based on age and risk factors. These discussions would likely have maintained recommendations for high-risk groups while potentially modifying guidance for lower-risk populations.
The scientific evidence supporting Covid-19 vaccination shows clear benefits for pregnant women, with studies documenting reduced hospitalization rates and improved outcomes for both mothers and infants. This evidence base has remained consistent throughout the pandemic and continues to support vaccination recommendations.
For children, the evidence picture is more complex, with studies showing that while children generally experience milder Covid-19 illness, they can still face serious complications. Hospitalization rates for children, while lower than for adults, still represent significant numbers of severely ill children.
The committee had also been reviewing data on myocarditis, a rare side effect associated with Covid-19 vaccination. Recent evidence showed extremely low rates of this complication, with no increased risk detected in the most recent monitoring period.
Scientific evidence continues to support vaccination
Despite Kennedy’s decision, the scientific evidence supporting Covid-19 vaccination for pregnant women and children remains robust. Multiple large-scale studies have documented the safety and effectiveness of these vaccines in these populations.
Research on pregnant women has consistently shown that Covid-19 vaccination reduces the risk of severe illness, hospitalization, and adverse pregnancy outcomes. These benefits extend to infants, who receive protective antibodies through maternal vaccination.
Pediatric studies have demonstrated that Covid-19 vaccines are safe and effective in children, with serious adverse events remaining extremely rare. The benefits of vaccination continue to outweigh the risks, even as Covid-19 severity has generally decreased compared to earlier phases of the pandemic.
Safety monitoring systems continue to track vaccine-related adverse events, with data showing that serious complications remain rare. The most concerning potential side effect, myocarditis, occurs at very low rates and is typically mild and resolves quickly.
The scientific consensus among medical professionals continues to support Covid-19 vaccination for pregnant women and children as an important tool for preventing serious illness and complications.
Impact on public health messaging and trust
Kennedy’s decision has created confusion and concern among healthcare providers and patients about appropriate medical care. The removal of recommendations without scientific justification contradicts established medical guidance and may undermine public trust in health authorities.
Healthcare providers now face the challenge of counseling patients about Covid-19 vaccination without clear government guidance. Many will likely continue to recommend vaccination based on the scientific evidence, but the mixed messaging from federal authorities complicates patient education efforts.
The decision may also affect international perceptions of American public health leadership, as other countries continue to recommend Covid-19 vaccination for pregnant women and children based on the same scientific evidence that remains available to American policymakers.
Public health advocates worry that the precedent set by bypassing scientific review processes could affect future policy decisions and undermine the role of expertise in protecting public health.
The controversy surrounding Kennedy’s announcement highlights ongoing tensions between political considerations and scientific evidence in public health policy, with potential long-term implications for how Americans approach vaccination and preventive healthcare.
As the medical community continues to advocate for evidence-based policy, the impact of this decision on public health outcomes will likely become clearer over time, particularly as it affects vaccination rates and health outcomes in the affected populations.